Intermediate
Product Center
News Information
Contact Us
Administrative Department landline 023-61213011
Securities Department landline 023-61213003
Postal Code 401121
Address: No. 2 Huangyang Road, Yubei District, Chongqing
| Product name | CAS NO. | Structure | Remark & Status | registration status |
| Specialized medication: | ||||
| Quetiapine Fumarate | 111974-72-2 | 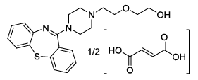 |
GMP/DMF Korea MFDSCommercial |
A |
| Rocuronium bromide | 119302-91-9 | 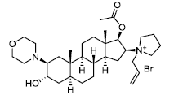 |
GMP/DMF Indian DCGI Commercial | A |
| L-ornithine L-aspartate | 3230-94-2 | 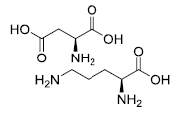 |
GMP/DMF Indian DCGI Commercial | A |
| α-Ketophenylalanine Calcium | 51828-93-4 | 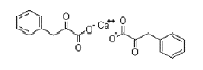 |
Commercial | A |
| Calcium alpha-ketovaline | 51828-94-5 | 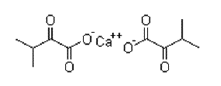 |
Commercial | A |
| α-Ketoleucine Calcium | 51828-95-6 | 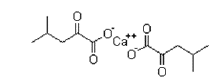 |
Commercial | A |
| D,L-α-Ketoisoleucine Calcium | 66872-75-1 | 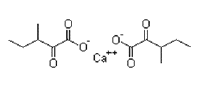 |
Commercial | A |
| D,L-α-Hydroxymethionine Calcium | 4857-44-7 | 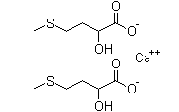 |
Commercial | A |
| Hydroxychloroquine sulfate | 747-36-4 | 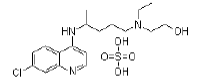 |
GMP Commercial |
A |
| Esomeprazole Sodium | 161796-78-7 | 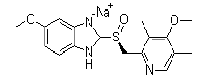 |
GMP Commercial |
A |
| Mosapride Citrate | 112885-42-4 | 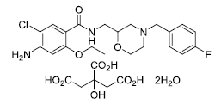 |
DMF Taiwan Commercial | A |
| Oxiracetam | 62613-82-5 | 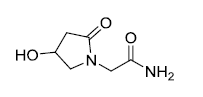 |
GMP Korea MFDS Commercial | A |
| Rebamipide | 90098-04-7 | 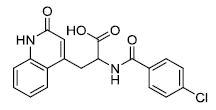 |
Korea MFDS Commercial | A |
| Febuxostat | 144060-53-7 | 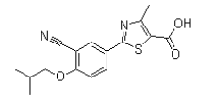 |
Commercial | A |
| Lercanidipine hydrochloride | 132866-11-6 | 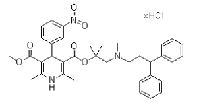 |
Commercial | A |
| Nicergoline | 27848-84-6 | 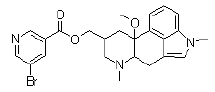 |
DMF Commercial |
A |
| Butorphanol Tartrate | 58786-99-5 | 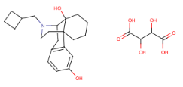 |
R&D | A |
| Tenofovir disoproxil fumarate | 202138-50-9 | 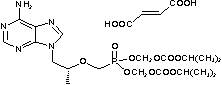 |
GMP Commercial |
A |
| Anti-Infective Drugs | ||||
| Tigecycline | 220620-09-7 | 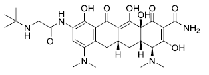 |
USDMF #27477 Commercial | A |
| Latamoxef Sodium | 64953-12-4 | 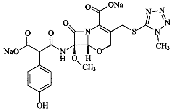 |
Commercial | A |
| Cefmetazole Sodium | 56796-39-5 | 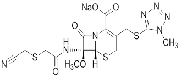 |
GMP/DMF Commercial |
A |
| Ceftizoxime sodium | 68401-82-1 | 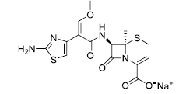 |
GMP/DMF Indian DCGI Commercial | A |
| Cefathiamidine | 33075-00-2 | 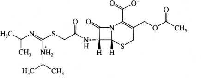 |
GMP Commercial | A |
| Cefotiam Hydrochloride | 66309-69-1 | 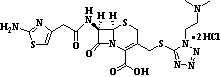 |
GMP Commercial | A |
| Clindamycin phosphate | 24729-96-2 | 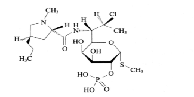 |
GMP Commercial | A |
| Aztreonam | 78110-38-0 | 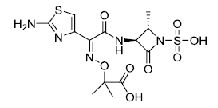 |
GMP USDMF#27508 Indian DCGI Commercial | A |
| Aztreonam And Arginine | ---- | 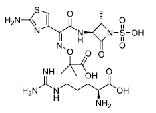 |
GMP/DMF Commercial |
A |
| Cefpirome sulfate | 98753-19-6 | 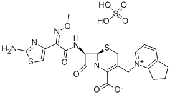 |
A | |
| Sulbenicillin Sodium | 28002-18-8 | 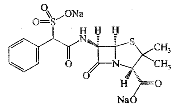 |
GMP Commercial |
A |
| Ornidazole | 16773-42-5 | 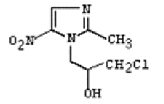 |
Commercial | A |
| Voriconazole | 137234-62-9 | 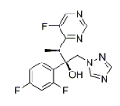 |
Commercial | A |
| Cefditoren pivoxil | 117467-28-4 | 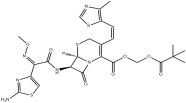 |
A | |
| Registered product: | ||||
| Nebivolol Hydrochloride | 152520-56-4 | 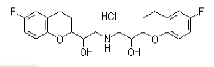 |
Commercial | I |
| Cinacalcet Hydrochloride | 364782-34-3 | 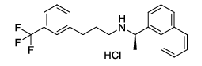 |
Commercial | I |
| Avibactam Sodium | 1192491-61-4 | 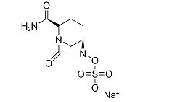 |
Commercial | I |
| Flomoxef Sodium | 92823-03-5 | 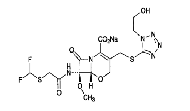 |
—— | |
| Oxacillin Sodium | 1173-88-2 | 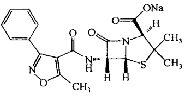 |
A | |
| Indobufen | 63610-08-2 | 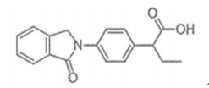 |
A | |
| Piribedil | 3605/1/4 | 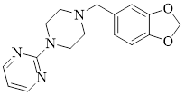 |
I | |
| Ranolazine | 95635-55-5 | 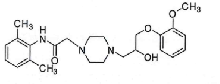 |
I | |
| Empagliflozin | 864070-44-0 | 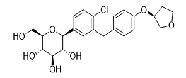 |
A | |
| Midazolam hydrochloride | 59467-96-8 | 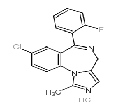 |
A | |
| Cefazolin Sodium Pentahydrate (Bosheng Label Template English: Cefazolin Sodium Hydrate) | 27164-46-1 | 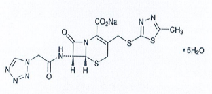 |
—— | |
| ☆☆☆ For foreign markets only | ||||
| Ticarcillin Sodium/Clavulanate Potassium | 30-1&15-1 | 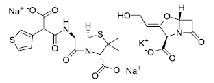 |
DMF Indian DCGI Commercial |
—— |
| Carbenicillin Sodium | 4800-94-6 | 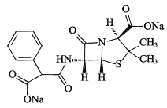 |
Commercial | —— |
| Cefsulodine Sodium | 52152-93-9 | 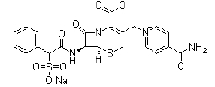 |
Commercial | —— |
| Product name | CAS NO. | Structure | Remark & Status | registration status |
| Diazoxide | 364-98-7 | 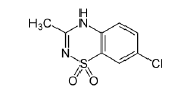 |
DMF USDMF#32771 Commercial |
DMF USDMF # 32771 can be jointly declared domestically |
| Trimeprazine tartrate | 4330-99-8 | 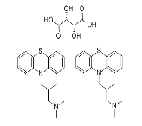 |
US VMF 6199 Commercial |
USDMF # 6199 can be jointly declared domestically |
| Fosphenytoin sodium | 92134-98-0 | 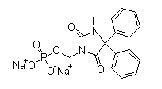 |
DMF USDMF#32816 |
DMF USDMF #32816 |
| Palonosetron Hydrochloride | 135729-62-3 | 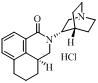 |
Commercial | Registration in China and the United States can provide associations |
| Dexmedetomidine hydrochloride | 145108-58-3 | 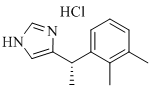 |
Commercial | Y20190000679 USDMF |
| Thioctic Acid | 1077-28-7 | 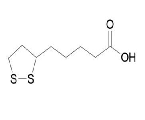 |
Commercial | Registration in China and the United States can provide associations |
| Product name | CAS NO. | Structure | Remark & Status | registration status |
| API | ||||
| Gemcitabine Hydrochloride | 122111-03-9 | 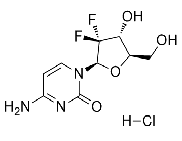 |
GMP/ DMF/ Commercial |
A |
| Granisetron Hydrochloride | 107007-99-8 | 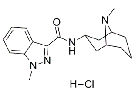 |
GMP Commercial |
A |
| Ondansetron Hydrochloride |
103639-04-9 | 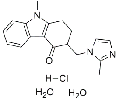 |
GMP Commercial |
A |
| Doxofylline | 69975-86-6 | 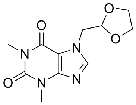 |
GMP Commercial |
A |
| Carvedilol | 72956-09-03 | 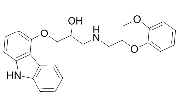 |
GMP Commercial |
A |
| Sulindac | 38194-50-2 | 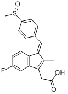 |
GMP Commercial |
A |
| Toremifene Citrate |
89778-27-8 | 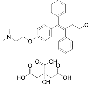 |
GMP/ PMDA Commercial |
A |
| Flumazenil | 78755-81-4 | 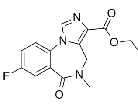 |
GMP Commercial |
A |
| Formoterol Fumarate |
43229-80-7 | 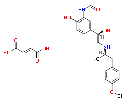 |
GMP Commercial |
A |
| Levocetirizine Hydrochloride |
130018-87-0 | 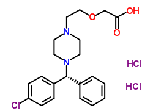 |
GMP Commercial |
A |
| Baclofen | 1134-47-0 | 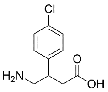 |
GMP Commercial |
A |
| Regorafenib | 755037-03-7 | 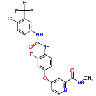 |
Commercial | A |
| Peramivir Trihydrate | 1041434-82-5 | 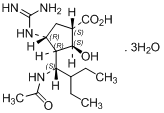 |
Commercial | A |
| Pamidronate disodium |
109552-15-2 | 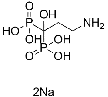 |
GMP Commercial |
A |
| Tofacitinib citrate | 540737-29-9 | 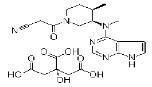 |
Commercial | A |
| Terbutaline sulfate | 23031-32-5 | 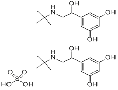 |
R&D | A |
| Product under development | ||||
| Marinidazole | 92478-27-8 | 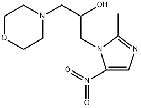 |
R&D | / |
| Noradrenanili Bitartas | 108341-18-0 | 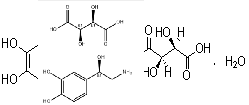 |
R&D | / |
| Elagolix Sodium | 832720-36-2 | 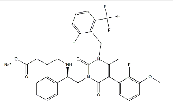 |
R&D | / |
| Revefenacin | 864750-70-9 | 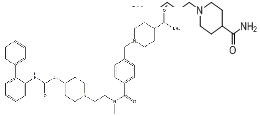 |
R&D | / |
| Azilsartan Medooxomil Potassium | 1417576-00-1 | 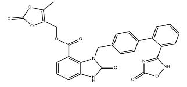 |
R&D | / |
| Pemafibrate | 848259-27-8 | 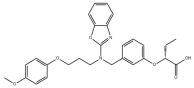 |
R&D | / |
| Bromhexine hydrochloride | 611-75-6 | 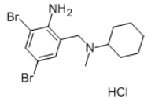 |
R&D | / |
| (±)-Evodiamine | 1722-62-9 | 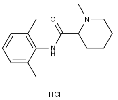 |
R&D | / |
| Cariprazine hydrochloride | 1083076-69-0 | 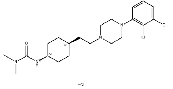 |
R&D | / |
| Gentamicin Sulfate | 1405-41-0 | 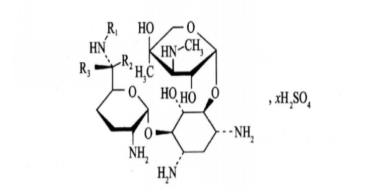 |
IndianFDA registration certificate, certificate number: RC/BD-002757; Korea FDA Registration Certificate, Certificate Number: 2020-A1-1266 | Registration number: Y20190002059 Registration status: A |




